Smart pill maker seeks FDA approval for new method of drug administration
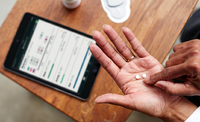
A new intelligent medication sensor has been brought to market for use together with existing medications, by the company Proteus Digital Health. Alongside pharmaceutical company Otsuka Pharmaceutical, Proteus has now sought approval from the FDA for distributing the ingestible smart sensor embedded inside newly manufactured medication.
How Does the Medication Smart Sensor Work?
The current, FDA-approved method that the medication smart sensor uses is a combination of an ingestible sensor device and a patch which is worn against the skin.
The sensor and patch collect data from the patient, and transmit it via Bluetooth to an app which can be accessed by the patient, their designated care team, and their doctor. The sensor itself uses a thin layer of metals which react with stomach acid in the patient’s stomach. This produces a small electrochemical reaction, which powers the sensor enough to send the data via Bluetooth. Currently, the sensor is swallowed alongside medication prescribed by the doctor.
Otsuka and Proteus recently took the next step, applying to the FDA for approval to actually embed the sensor inside the medication itself. In particular, they applied for approval in relation to the drug aripiprazole, which is an antipsychotic used to treat schizophrenia and bipolar disorder.
What Is the Aim of the Smart Sensor?
The primary use of the smart sensor is to provide two types of information: one set of information confirms to the doctor that the patient has swallowed their medication; the second set provides biometric data about the patient’s physiological state after they have taken the medication (e.g. heart rate, blood pressure, and temperature).
In some cases, tracking the patient’s response to the medication is vitally important, particularly when considering the number of pharmacy errors that occur each year: in the UK, over 10,000 medication mistakes are self-reported by pharmacists each year. The causes of pharmacy errors are numerous, with a medical law firm finding that claims are most often for:
- Incorrect prescription being provided to the patient
- Expired medication being given
- Incorrect medication being given
- Improper mixing of medication components, or incorrect amounts of medication components
- Unclear or incorrect instructions on medication
This means that the Proteus sensor could be used to quickly flag when a medication mistake has occurred, such as an incorrect medication being prescribed and causing an adverse reaction.
In other cases, the evidence that the patient has actually taken their medication is more important for the care provider. For example, in cases of dementia or mental illness the medication may simply be forgotten or may be purposefully avoided. This is the issue that Proteus and Otsuka hope to solve with their next step of approval from the FDA.
Smart medication sensors like the one produced by Proteus could be used to prevent medication adherence problems, and the next step towards actually embedding the smart sensor inside a pill would go a long way towards alleviating the issues caused by patients who simply avoid taking their medication or only pretend to take it. In these types of cases, both Proteus and Otsuka “believe this new digital medicine could revolutionize the way adherence is measured and fulfil a serious unmet medical need in this population.”
It currently looks like the FDA has rejected approval for embedding the sensor inside medication, however the device/drug combination will be able to be resubmitted to the FDA if Proteus and Otsuka choose to do so.

